Is it safe to start bioidentical hormones after menopause?
Dr. Karen Plymel, Functional Medicine Expert

Yes, for many women, it can be safe—depending on timing, formulation, and individual risk factors. The key is understanding how different hormones, routes of administration, and timing affect safety and outcomes.
Background: Why the NIH (WHI) Study Raised Alarm
The NIH Women’s Health Initiative (WHI), launched in the 1990s, was the largest randomized controlled trial studying hormone replacement therapy (HRT) in postmenopausal women. Here’s what it found:
Subjects: Mostly women who were over 60 and years past menopause.
Hormones studied:
- Conjugated equine estrogens (CEE) (from horse urine)
- Medroxyprogesterone acetate (MPA) (a synthetic progestin)
Results: Increased risk of:
- Heart disease
- Stroke
- Breast cancer
- Blood clots
These risks led to a dramatic drop in HRT use and a general fear around it. However, subsequent analysis revealed that age and timing were major confounders. Women who started HRT close to menopause (within 10 years) tended to have neutral or even beneficial effects on heart health.
Bioidentical vs Synthetic Hormones: What's the Difference?
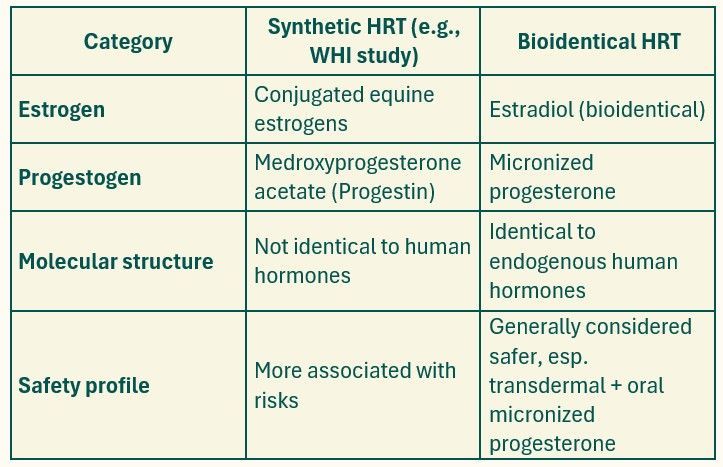
Transdermal estradiol (e.g., patch, gel) and oral micronized progesterone (e.g., Prometrium) are bioidentical and have favorable safety data , especially regarding clot risk, breast cancer risk, and cardiovascular outcomes.
❤️ Cardiovascular Health & Hormone Therapy
What We Know from Later Studies:
1. KEEPS Trial (Kronos Early Estrogen Prevention Study)
- Studied women within 3 years of menopause
- Used transdermal estradiol and oral micronized progesterone
- Showed no adverse effects on cardiovascular markers
- Some positive effects on carotid artery thickness (early atherosclerosis)
2. ELITE Trial (Early vs. Late Intervention Trial with Estradiol)
- Compared early (≤6 years postmenopausal) vs. late (≥10 years) estradiol initiation
- Found that early initiation slowed progression of atherosclerosis
- No benefit when started late
3. Danish Osteoporosis Prevention Study (DOPS)
- Over 1000 women, treated with HRT shortly after menopause
- After 10 years: Significant reduction in heart disease and no increase in breast cancer
- This was not using bioidentical estradiol , but supports early timing.
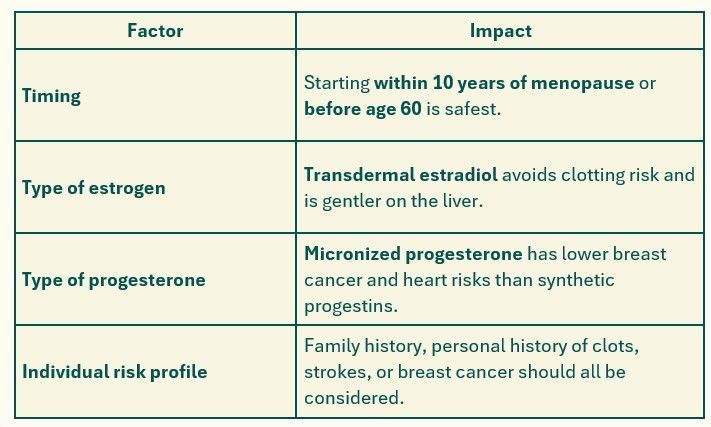
So, Are Bioidentical Hormones Safe After Menopause?
When started early (within 10 years of menopause) and using bioidentical formulations , there’s good evidence they are safe and beneficial , particularly for:
- Cardiovascular health
- Bone density
- Cognition
- Mood and sleep
- Vaginal and urinary symptoms
Bottom Line
- The WHI study showed risks from synthetic hormones given to older women long after menopause.
- Bioidentical hormones, when started early are much safer —especially transdermal estradiol and oral micronized progesterone.
- Work with a functional medicine provider to assess personal risks and monitor therapy carefully.